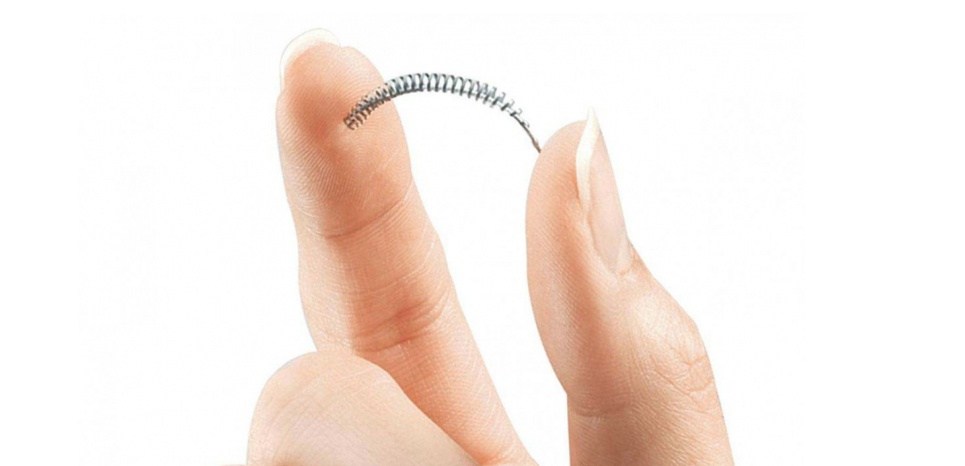
Essure is the only non-surgical sterilization device on the market. It was advertised as a quick outpatient procedure with a fast recovery time for busy women. Instead, over 5,000 women have experienced severe side effects that could only be cured with a hysterectomy.
One of these women told NPR, “It feels like you’ve been hit by a truck every day of your life. For me, it’s been a nightmare. I mean, this device literally ruined my life.”
Another woman told ABC News, “My whole body started to change. … I was itchy, my arms were tingling and my legs were tingling. … I was confused all the time.”
Last year, a study published in the British Medical Journal associated Essure with 2.9% risk of needing follow-up surgery — approximately 10-times the 0.2% risk in women who undergo traditional “tube tying” surgery.
In February 2016, the FDA ordered new clinical trials and updated the label to include a “Black Box” warning about severe side effects, which may include:
- Chronic pain
- Perforation of the uterus or fallopian tubes
- Device fracture
- Migration
- Heavy bleeding
- Allergic reactions to nickel
- Fatigue
Essure is a matchstick-sized device that is inserted through the vagina into the fallopian tubes. The device is wrapped in two metal coils, one made of a nickel-titanium alloy and another covered in an irritating polyester substance that causes inflammation. In a few months, scar tissue blocks the fallopian tubes and prevents pregnancy.
Until recently, Bayer claimed immunity from lawsuits based on a loophole in the way Essure was approved. The lawsuits did not begin moving forward until Erin Brokovich began campaigning to change preemption laws.
Bayer is now facing over 100 lawsuits involving Essure in Canada. In the United States, at least five lawsuits are slowly making their way through the court system.
Source: Essure Procedure