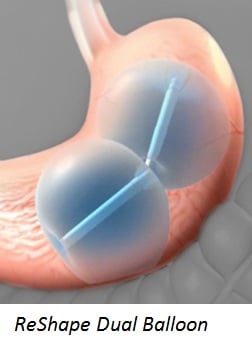
ReShape® Integrated Dual Balloon System (Reshape Medical)
The FDA issued an update to alert healthcare providers of 5 deaths that occurred since 2016 in patients with liquid-filled stomach balloons.
Four deaths involve the Orbera® Intragastric Balloon System made by Apollo Endosurgery and 1 death involves the ReShape® Integrated Dual Balloon System manufactured by Reshape Medical.
All 5 patients died within 1 month of receiving the weight-loss balloon. Three of the patients died just 1-3 days after the balloon was placed.
Two additional deaths since 2016 were related to potential complications — one stomach perforation due to Orbera, and one esophageal perforation with the ReShape weight-loss balloon.
According to the FDA:
At this time, we do not know the root cause or incidence rate of patient death, nor have we been able to definitively attribute the deaths to the devices or the insertion procedures for these devices (e.g., gastric and esophageal perforation, or intestinal obstruction).”
Today’s FDA warning follows another alert in February 2017, after dozens of patients suffered life-threatening side effects when their balloon spontaneously over-inflated with air or fluid.
The FDA also warned that several patients developed acute pancreatitis (pancreas inflammation) because the fluid-filled balloons compressed their internal organs. Four patients were hospitalized.
Most of the adverse events involve Obera, which uses saline to fill a single stomach balloon, as opposed to the ReShape system that uses 2 balloons filled with saline and a blue dye. Both systems can be placed in the stomach for up to 6 months to help obese people lose weight.
The symptoms of balloon over-inflation or pancreatitis include severe abdominal or back pain, abdominal swelling, trouble breathing, fever or vomiting. Pancreatitis and balloon over-inflation were not listed as potential side effects until February 2017, so doctors might not be aware of the risk.
Source: Liquid-filled Intragastric Balloon Systems: Letter to Healthcare Providers – Potential Risks
