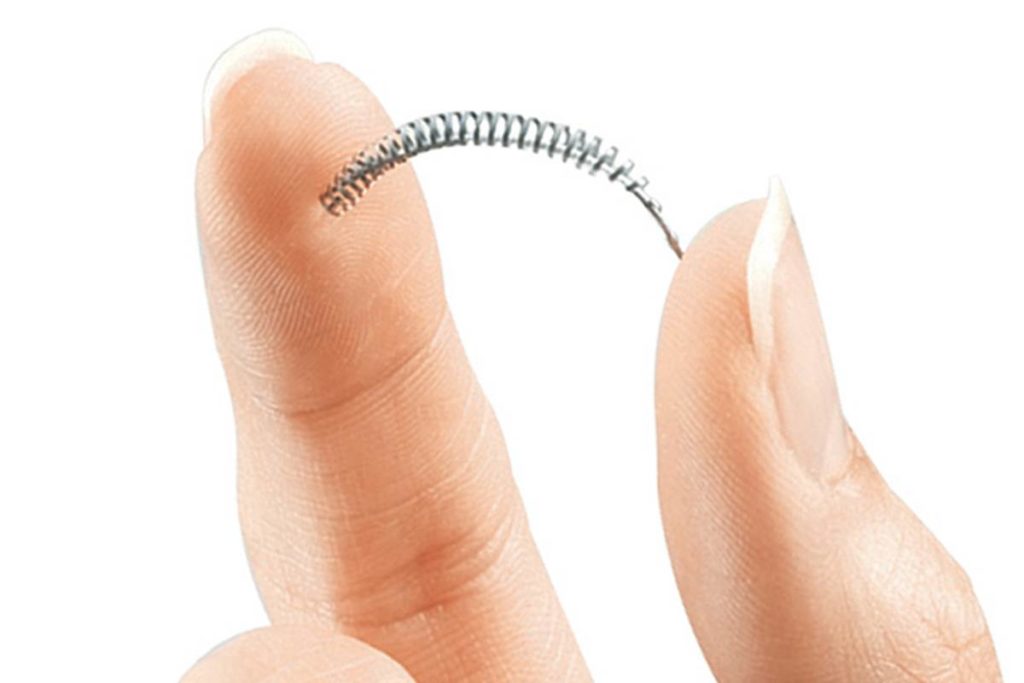
Essure is a type of permanent birth control. Matchstick-sized metal coils are inserted through the vagina into both fallopian tubes, where irritating fibers of PET trigger inflammation. Over the next 3 months, scar-tissue blocks the fallopian tubes to prevent pregnancy.
Bayer advertised Essure as a quick 30-minute non-surgical outpatient procedure for busy women, with less pain and a faster recovery time than a traditional “tube tying” or tubal ligation sterilization procedure.
Instead, studies have shown that women who choose Essure are twice as likely to require follow-up surgery. In the U.S., over 10,000 women have reported serious long-term side effects from Essure.
This week, Bayer told The Toronto Star that it will stop selling Essure in Canada over the next few months due to lack of demand:
This decision was taken for commercial reasons, and the favourable benefit-risk profile of Essure remains unchanged. This is not a recall of the product from the market.”
Earlier this year, Health Canada issued an advisory to warn that some patients were not adequately warned about the risks of Essure. These warnings followed similar warnings from the FDA.
The risks associated with Essure include bleeding, unintended pregnancy, ectopic pregnancy, chronic pain, auto-immune disease, organ damage or perforation, migration of the device, fracture, allergic reactions to nickel, and ongoing immune-type reactions.
Source: Controversial Essure birth control device will no longer be sold in Canada