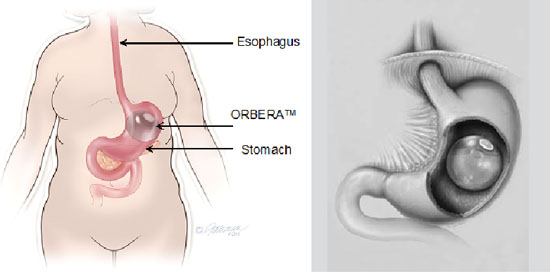
The Orbera Balloon System is inserted into a patient’s stomach and filled with saline (salt-water). The ReShape Integrated Dual Balloon System uses two balloons that are filled with saline and blue dye.
In a letter to doctors on February 9, the FDA described two serious side effects of fluid-filled gastric balloons that are used to treat obesity.
The first side effect is balloon over-inflation. The FDA reported “several dozen” injuries (mostly with the Orbera Balloon, but also some with the ReShape System) as soon as 9 days after implantation.
Healthcare providers and Emergency Room (ER) doctors may not realize these symptoms are related to a weight-loss balloon because spontaneous over-inflation is not on the label for ReShape or Orbera.
The second side effect is acute pancreatitis — sudden inflammation of the pancreas — due to compression of organs by a stomach balloon. It can occur within 3 days of implantation.
Four patients had to be hospitalized, but several more were injured. All of these patients had to have their stomach balloons removed. The primary symptoms included severe abdominal pain and back pain.
Pancreatitis is not on the list of ReShape or Orbera side effects, so doctors and ER physicians may not be aware of the risk. This could lead to misdiagnosis, unsuitable treatment, or delayed treatment of pancreatitis until the disease becomes life-threatening.
The FDA is working with the manufacturers — ReShape Medical Inc. and Apollo Endo-Surgery — to better understand these issues. In the meantime, the agency is asking doctors to closely monitor patients and report serious problems to MedWatch.
Source: FDA warns on over-inflation, pancreatitis risks with ReShape, Apollo intragastric balloons