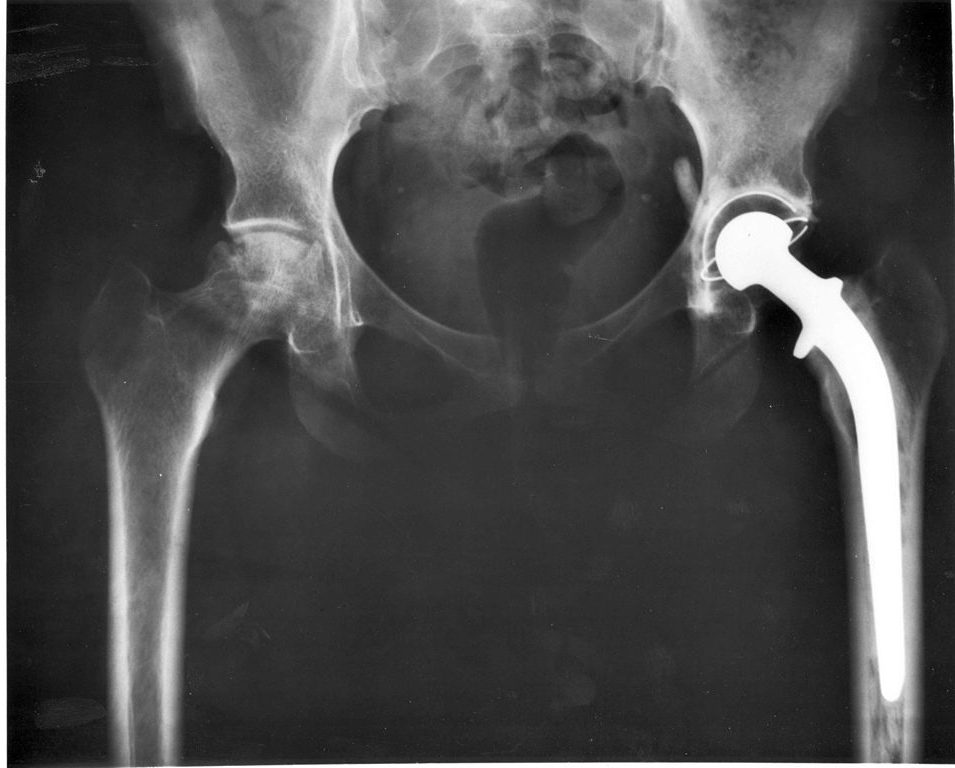
Australian officials issued a hazard alert for Stryker hip implant taper lock failures in August 2016.
In November, the FDA announced a recall for certain Stryker LFIT V40 Femoral Heads — a metal “ball” part of the hip joint — manufactured from 2002 to 2011.
The LFIT V40 is designed to lock onto a femoral hip stem with a metal part called a trunnion. This creates a problematic metal-on-metal connection that can corrode and break within a few years.
Corrosion is a major problem with metal hip implants. Metal debris in the hip joint can cause pain, inflammation, soft-tissue growths called pseudotumors, or get into the bloodstream and poison the body.
In January 2017, a lawsuit was filed by a man from Alaska who developed all of these side effects and had to have another surgery.
The man, Patton Witt, was implanted with an LFIT V40 in combination with a Stryker Accolade TMZF femoral stem in March 2008. After his revision surgery in January 2015, doctors wrote:
One could clearly see extensive corrosion present at this site. There appeared to be some deterioration at the trunnion with loss of the passified layer.”
The lawsuit was filed in federal court in Alaska on January 12, 2017 — In RE: Patton Witt vs. Howmedica Osteonics Corp. — Case No. 4:17-cv-00001.
Taper lock failures, also known as trunnionosis, are recognized as a growing cause of hip replacement failures. There is no good solution.
Revision surgery is physically traumatic, especially for older patients. The old implant must be removed and a new metal stem must be pounded into the femur. Many patients are left with one leg shorter than the other.
High levels of cobalt in the bloodstream from failed hip replacements can also cause brain damage, low thyroid problems, and heart disease (cardiomyopathy) that may not resolve after removal of the implant.
The severity of these side effects is why thousands of people have filed lawsuits against manufacturers of metal hip replacements.
Stryker has already paid $1 billion in settlements to 6,000 people who were injured by another metal hip implant that corroded — the recalled the ABG II and Rejuvenate hips. The settlement provided base payouts of $300,000 per hip replacement that required surgery.
Source: Stryker LFIT V40 Hip Replacement Lawsuits May Be Centralized In Single Federal Court