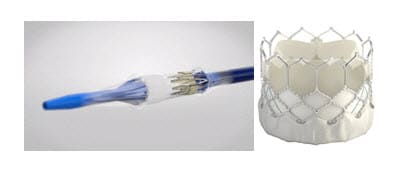
Edwards SAPIEN 3 Ultra Delivery System
The recall involves the Sapien 3 Ultra delivery system manufactured by Edwards Lifesciences Corp. The system is used during open-heart surgery to remove and replace a diseased heart valve.
Two months ago, Edwards sent a safety notice to customers, warning that the balloon in the delivery system can burst. They recommended inflating the balloon slowly and continuously to avoid rupture.
When the balloons burst during implantation procedures, it can be extremely difficult to retrieve the heart valve in the catheter and withdraw the system from a patient’s body, according to the FDA. Doing so can cause vascular injury, bleeding, or surgical intervention, which may result in death.
As of July 2019, a total of 17 injuries and at least 1 death have been linked to burst balloons on the device.
The FDA has designated this a “class I recall,” which is the most serious type of recall the agency can issue.
It is reserved for situations in which the FDA believes that use of an affected product may cause serious adverse health consequences, including death.