FDA says consumption of the recalled product could increase the “remote probability of necessitating medical or surgical intervention to preclude or reverse permanent damage to a body structure or function.”
Life-Line Water was distributed nationwide via Internet sales in 1-gallon bottles, FDA said.
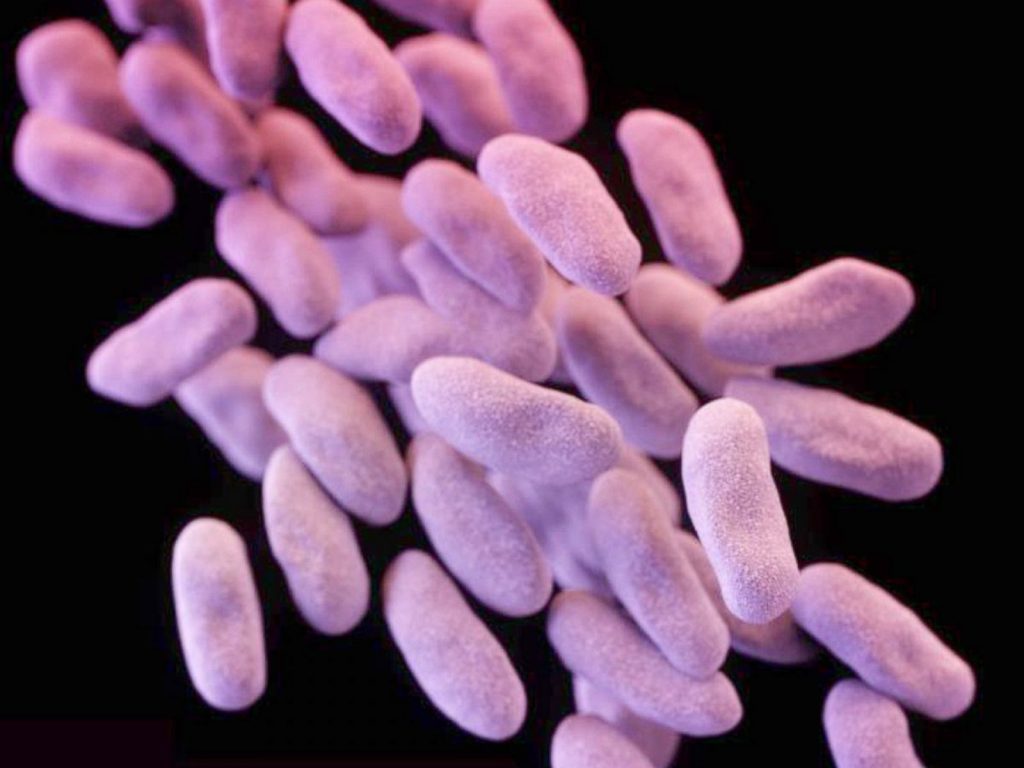
According to the U.S. Centers for Disease Control and Prevention (CDC), the most serious infections of P. aeruginosa typically occur in hospitalized patients, and in individuals with significantly reduced immune systems. Infections of the blood, pneumonia, and infections following surgery can lead to severe illness and even death in these people.
Signs and symptoms of of P. aeruginosa vary based on the type of infection.
Bloodstream infections can cause:
- Fever and chills
- Body aches
- Light-headedness
- Rapid pulse and breathing
- Nausea and vomiting
- Diarrhea
- Decreased urination
Pneumonia can cause:
- Fever and chills
- Difficulty breathing
- Cough, sometimes with yellow, green, or bloody mucus
Urinary tract infections can cause:
- Strong urge to urinate frequently
- Painful urination
- Unpleasant odor in urine
- Cloudy or bloody urine
Wound infections can cause:
- Inflamed wound site
- Fluid leakage from wound
Ear infections can cause:
- Ear pain
- Hearing loss
- Dizziness and disorientation
To date, FDA said it is unaware of any bacterial infections or other injuries related to the recalled Life-Line Water additive.
Source: WTOL