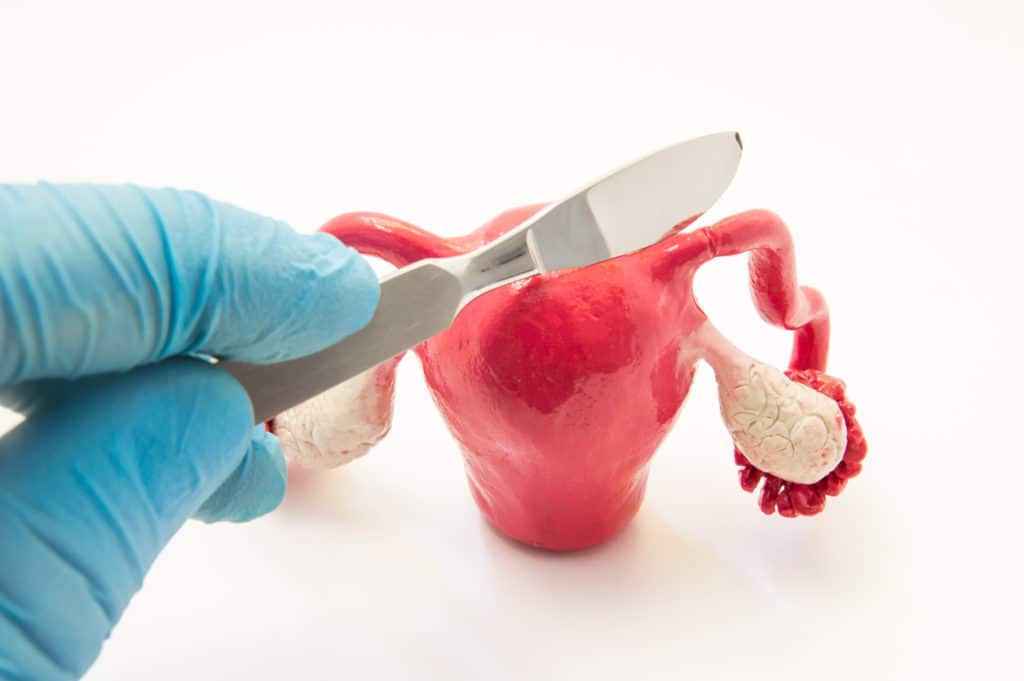
Essure is a medical device that is inserted into a woman’s reproductive system, where it triggers inflammation that is supposed to seal off the fallopian tubes and prevent pregnancy.
Bayer advertised Essure as a quick way for women to avoid pregnancy without undergoing a traditional tubal ligation surgery.
Instead, the company is facing around 18,000 lawsuits from women who suffered devastating complications. In most cases, these women had to undergo surgery to remove Essure.
On April 24, the FDA updated the Problems With Essure website to include data from more than 15,000 new adverse event reports in 2019.
Essure was recalled in most countries internationally in 2017, but Bayer continued to sell it in the U.S. until voluntarily pulling it off the market in December 2018, citing declining sales.
Essure may not be on the market anymore, but over 500,000 women in the U.S. have been implanted with the device — and thousands of them are developing long-term complications.
Bayer is required to keep track of adverse events associated with Essure and report them to the FDA on a quarterly basis.
Overall, there have been 47,856 total reports of adverse events from Essure’s approval in 2002 through 2019. Around 90% of those reports involve removal of the device.
From 2002 through 2019, the most frequently-reported patient problems were: pain/abdominal pain, heavier menses/menstrual irregularities, headache, foreign body/device fragment in patient, perforation, fatigue, weight fluctuations, depression/anxiety, hypersensitivity/rash, and hair loss.
The most frequent device problems were: patient-device incompatibility/biocompatibility (for example, possible nickel allergy or patient’s anatomy related to failure), migration of the device or device component, device breakage/material fragmentation/fracture, dislodged or dislocated device, device operating differently than expected (for example, implant failure or pregnancy), malposition of the device, device difficult to remove, or device difficult to insert.
Source: FDA Updates Essure Adverse Events Page to Include 2019 Reports