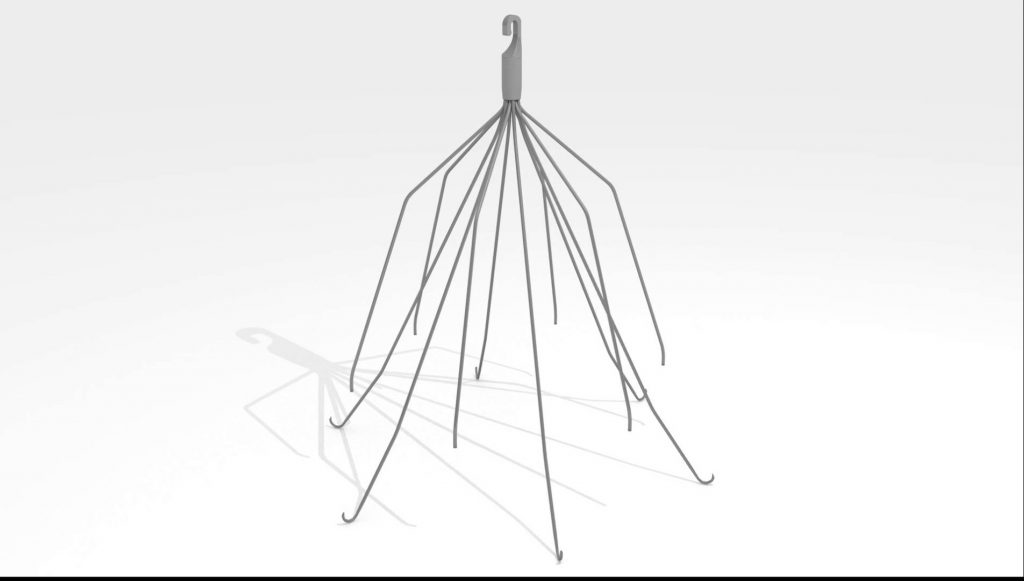
The lawsuit was filed by Juliette B., a woman from Florida who was implanted with the Meridian® Vena Cava Filter in September 2012.
She accuses C.R. Bard of negligence for selling a defective medical device, failing to warn about side effects, and concealing safety risks.
Meridian is a temporary filter that is implanted in the inferior vena cava (IVC). It catches blood clots before they travel to the lungs and cause a pulmonary embolism.
The problem is that the Meridian is very similar to other IVC filters made by C.R. Bard — including the G2 and Recovery — which have been linked to a 12% fracture risk in recent studies.
The FDA now recommends removing temporary IVC filters within 29-54 days. The longer they remain implanted, the higher the risk of complications.
The case was consolidated in a Multi-District Litigation (MDL No. 2641) where at least 810 lawsuits are pending against C.R. Bard. Another 800 lawsuits are pending in a similar litigation against Cook Medical.
The lawsuit was filed on August 19 in the U.S. District Court for Arizona, In re: Bard IVC Filters Products Liability Litigation — Case No. 2:16-CV-02791.
The plaintiff is represented by Ben C. Martin of the Law Offices of Ben C. Martin in Dallas, Texas.