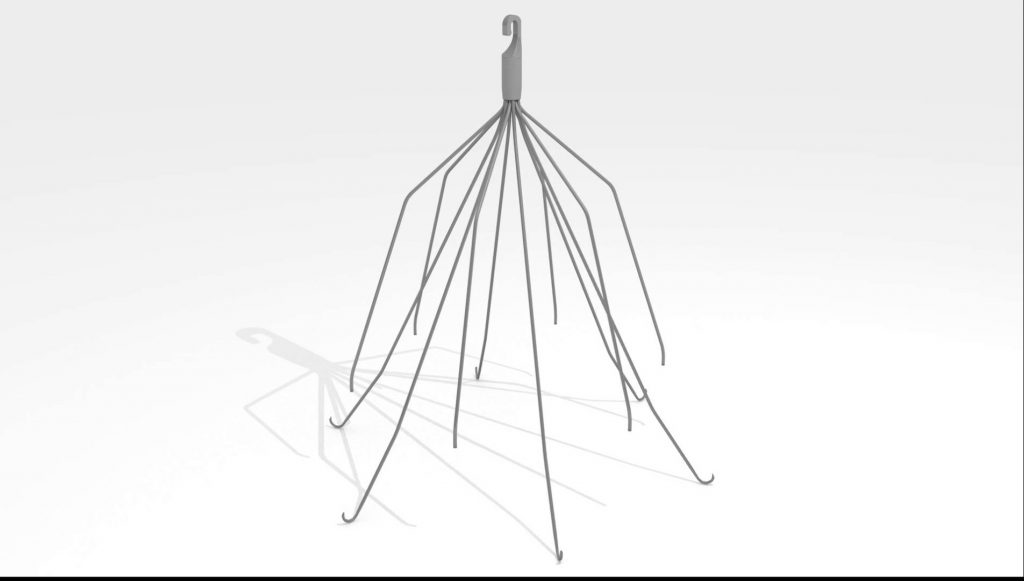
The plaintiff, Renee H., is a woman from New Jersey who was injured by the Meridian® Vena Cava Filter made by C.R. Bard and Bard Peripheral Vascular, Inc.
Meridian is a temporary filter that is placed in a major blood vessel called the inferior vena cava (IVC). It is designed to catch blood clots before they get to the lungs and cause a pulmonary embolism.
Like all retrievable IVC filters, the risk of complications increases dramatically the longer it remains implanted. After over 900 adverse events were reported, the FDA issued several Safety Warnings and recommended removing vena cava filters within 29-54 days.
The design of the Meridian is very similar to other filters made by Bard — including the G2 and Recovery — that have been linked to a 12% fracture risk in recent studies.
C.R. Bard is accused of negligence for creating and selling a defective medical device, failing to warn about side effects, fraudulent misrepresentation and concealment, and breaching implied and express warranty.
The company is facing over 600 federal lawsuits in Multi-District Litigation (MDL No. 2641) under District Judge David G. Campbell. The cases have been centralized in Arizona federal court for pretrial proceedings.
The lawsuit was filed on June 20, 2016 in the U.S. District Court for Arizona — Case No. MD-15-02641.
The plaintiff is represented by Ben C. Martin of the Law Offices of Ben C. Martin in Dallas, Texas.