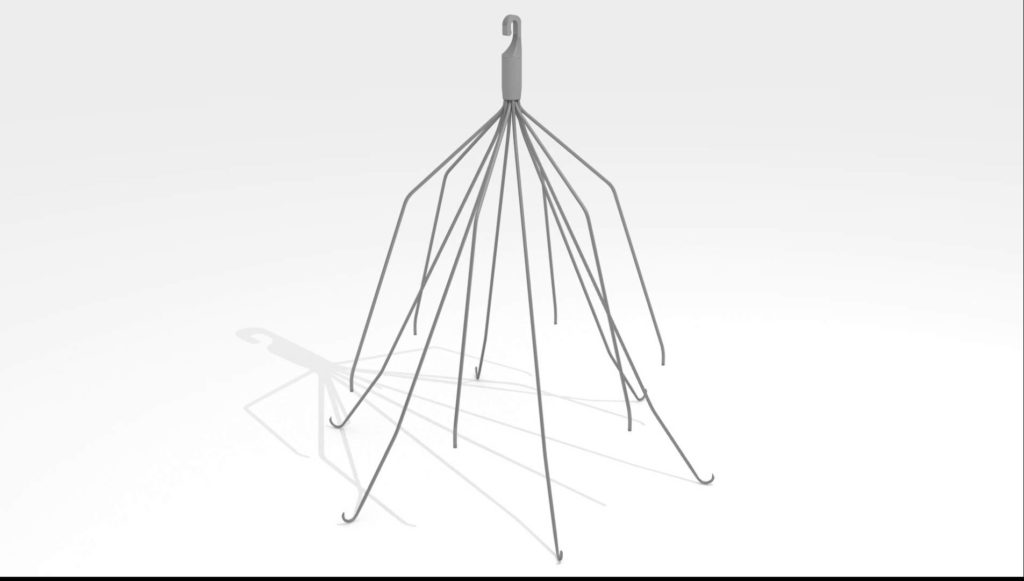
The lawsuit was filed by Jessica G., a woman who was implanted with the C.R. Bard Eclipse® Vena Cava Filter on June 6, 2011 at a hospital in New York.
She accuses C.R. Bard of negligence for selling a defective medical device and failing to warn about safety risks.
Eclipse is a retrievable blood clot filter that was approved in January 2010 with a 510(k) clearance, which means it did not go through clinical trials because it was “equivalent” to other filters on the market.
Eclipse is “equivalent” to the G2 Express, also made by C.R. Bard, but it has a more highly-polished surface. Soon after Eclipse was approved, Bard raised prices and pulled the G2 off the market.
Lawyers say the G2 may have similar design flaws as its own “equivalent” device — Bard Recovery® — which was pulled off the market in 2005 and later linked to a 40% five-year fracture risk.
In November 2010, a study of the G2 and the Recovery found high rates of filter fracture with fragment embolization to the heart.
In February 2012, another study found an overall 12% fracture rate out of 548 patients implanted with the G2 or Recovery. Only about 50% of the fractured components were successfully removed.
The lawsuit was filed on October 19, 2016 in the U.S. District Court for the District of Arizona — Case No. 2:16-cv-03615.
It will be centralized with over 1,000 other IVC filter lawsuits now pending against C.R. Bard in Multi-District Litigation (MDL No. 2641)— In Re: Bard IVC Filters Products Liability Litigation.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas.