The lawsuit was filed by eight people who were injured by the Cordis TrapEase® Permanent Vena Cava Filter or the Cordis OptEase® Retrievable Vena Cava Filter.
The lawsuit cites several studies linking OptEase and TrapEase filters with high rates of blood clots, fracture, and other major complications experienced by the plaintiffs:
OptEase filters and the TrapEase filters suffer fracture rates of 37.5% and 23.1% respectively, when left implanted a minimum of 46 months. Another recent study found that the TrapEase filter had a 64% fracture rate when left in more than four years.
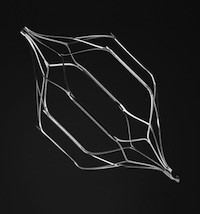
Cordis OptEase® Retrievable IVC Filter
Plaintiff Alice J. is a resident of Texas who was implanted with the OptEase IVC Filter on September 19, 2014. The filter tilted and become embedded in the wall of her vena cava. She suffered a failed retrieval attempt that led to further injuries, pain, and swelling.
Plaintiff Brenda M. is a resident of Texas who was implanted with the TrapEase IVC Filter on June 17, 2005. The filter fractured and perforated through the wall of her vena cava, causing life-threatening injuries and extensive medical care and treatment.
Plaintiff Amy L. is a resident of Arizona who was implanted with the OptEase IVC Filter on February 1, 2007. The filter malfunctioned and fractured. The broken pieces of the filter migrated into her lungs.
Plaintiff Diane Y. is a resident of Texas who was implanted with the TrapEase IVC Filter on January 15, 2006. The filter dislodged and migrated, causing significant medical expenses, pain, and suffering.
Plaintiff Joseph D. is a resident of Florida who was implanted with the TrapEase IVC Filter on January 12, 2008. The filter caused extensive Deep Vein Thrombosis (DVT), blood clots, clotting and occlusion of the vena cava, clotting of the extremities, and thrombolysis.
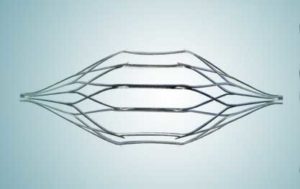
Cordis TreapEase® Permanent IVC Filter
Plaintiff Christopher H. is a resident of Texas who was implanted with the TrapEase IVC Filter on January 21, 2011. The filter perforated through the wall of his vena cava, in close proximity to the intestines and threatening the aorta.
Plaintiff John M. was a resident of Montana who is now living in California. He was implanted with the OptEase IVC Filter on January 19, 2009. The filter tilted, embedded in the wall of the vena cava, and was unable to be retrieved.
Plaintiff Stephanie E. is a resident of Illinois who was implanted with an OptEase IVC Filter on February 12, 2004. The filter migrated, fractured, and was unable to be removed despite two retrieval attempts.
Lawyers accuse Cordis Corporation, Johnson & Johnson, Cardinal Health Inc., and Confluent Medical Technologies of downplaying these serious safety risks.
The defendants are charged with 7 counts of strict products liability, failure to warn, manufacturing defect, negligence, fraudulent misrepresentation and concealment, and breach of express warranty.
The lawsuit was filed on April 28, 2017 in the Superior Court of the State of California (Alameda County) — Case No. RG1785842
There are now over 3,000 IVC filter lawsuits pending in state and federal courtrooms nationwide. The manufacturers include Cordis Corp., C.R. Bard, Cook Medical, B. Braun, Rex Medical, and more.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas.
Ben C. Martin is a trial attorney who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL and on the plaintiffs’ steering committee of the Bard IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

Just quick reminder
In 2011, FDA denied Citizen Petition FDA-2009-P-0362 proposing the chemical material test for nitinol. If had been implemented, this test could have greatly minimized the problem of nitinol IVC filters fractures. The test was rejected not on the ground of its inefficiency but on the ground of prevention of overburdening manufacturers of nitinol products.