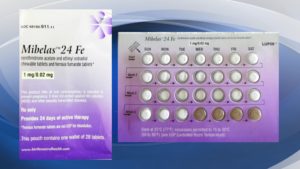
A customer complaint has identified a packaging error in which the blister was rotated 180 degrees in the wallet, reversing the weekly tablet orientation and making the lot number and expiration date no longer visible.
The first four days of the blister pack have four non-hormonal placebo tablets as opposed to the active tablets. As a result, pills that are taken out of sequence may place the user at risk for contraceptive failure and/or unintended pregnancy, according to an FDA Recall Notice issued May 25.
Affected products are packaged in blister packs containing 28 tablets: 24 white to off-white tablets of active ingredients debossed with “LU” on one side and “N81” on the other; and 4 tablets of inert ingredients debossed with “LU” on one side and “M22” on the other side.
The tablets were distributed to wholesalers, clinics and retail pharmacies across the U.S.
Customers who purchased Mibelas 24 Fe birth control tablets affected by this recall should notify their physician and return the product to the pharmacy or place of purchase.
Source: FOX31 Denver