The Brazilian health agency Anvisa recalled Essure on February 17, according to a notice posted on its website in Portuguese. The translation reads:
The contraceptive system is classified as maximum-risk. It can cause changes in menstrual bleeding, unwanted pregnancy, chronic pain, perforation and migration of the device, allergy and sensitivity, or immune-type reactions.”
Anvisa did not say how many Brazilian women received Essure, but Bayer estimates 900,000 devices have been sold worldwide, with at least 70% sold in the United States.
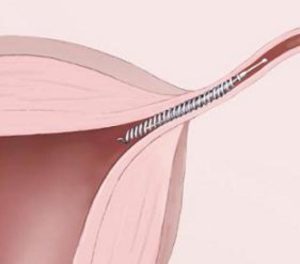
Essure is advertised as a quick 30-minute outpatient procedure for busy women, with less pain and faster recovery than a traditional “tube-tying” sterilization surgery known as tubal ligation.
This marketing campaign resulted in a skyrocketing number of women who chose Essure over a tubal ligation — and the number of injuries.
The FDA received 9,900 side effect reports, including 26 deaths and hundreds of pregnancy losses, between Essure’s approval date in November 2002 and December 2015.
The campaign to recall Essure in the U.S. started online with 30,000 “E-Sisters” on Facebook and gained momentum from Erin Brockovich and lawmakers. Over 900 women have joined lawsuits in California, with lawyers aggressively challenging Bayer’s claim of legal immunity.
However, the FDA rejected demands to recall Essure last year, leaving many women questioning what it would take. Instead, Essure got a “Black Box” warning label and an optional “Patient Decision Checklist” — which, to be fair, would be frightening to read if doctors actually gave it out.
Essure is a Class III (high-risk) device that is only necessary for a tiny number of women who can’t have surgery and choose to be sterilized. The FDA said Essure “remains an appropriate option for the majority of women seeking a permanent form of birth control,” but “some women may be at risk for serious complications.”
The FDA also asked Bayer to study how the risks of Essure compare to tubal ligation. It will be years before Bayer’s study is complete, but in 2015, a study of 52,000 women in New York estimated that women with Essure were 10-times more likely to need follow-up surgery compared to women who had a tubal ligation.
