Viberzi (eluxadoline) is a twice-daily pill sold by Allergan for the prevention of diarrhea in adults with Irritable Bowel Syndrome (IBS).

This is a good way to stop diarrhea. The problem is that Viberzi might also slow down other muscles that control the flow of digestive juices into the intestines.
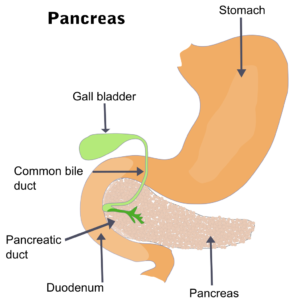
One of those muscles is the sphincter of Oddi, a ring-shaped valve that opens and closes the bile duct. If it does not open, digestive juices will get backed up in the gallbladder and pancreas.
In people without a gallbladder, there is nowhere for backed-up juices to go except the pancreas. As the enzymes start digesting the pancreas, it can cause inflammation (pancreatitis), excruciating pain, or even death.
People without gallbladders should not use Viberzi due to the risk of pancreatitis and death, according to a Drug Safety Communication from the FDA.
The FDA received 120 reports of severe pancreatitis or death from the time Viberzi was approved in May 2015 through February 2017, including 76 people who were hospitalized.
At least 56 victims did not have a gallbladder, including 2 people who died. The FDA warned:
Hospitalizations and deaths due to pancreatitis have been reported with Viberzi use in patients who do not have a gallbladder. Symptoms of pancreatitis have occurred with just one or two doses of Viberzi.”
Patients should stop using Viberzi and get emergency medical care for new or worsening stomach pain, or pain in the upper-right side that radiates to the back or shoulder, sometimes with nausea or vomiting. These are symptoms of pancreatitis and sphincter of Oddi spasms.
- Bismuth subsalicylate (Kaopectate and Pepto-Bismol)
- Loperamide (Imodium)
- Diphenoxylate/ atropine (Lomotil)
- Simethicone (Gas-X, Mylicon)
- Alosetron hydrochloride (Lotronex)
- Rifaximin (Xifaxan)

