The lawsuit was filed by Terry P. Jr., a man from Florida who was implanted with the Cook Günther Tulip® Vena Cava Filter (“IVC Filter”) on January 26, 2007 at University Community Hospital in Tampa.
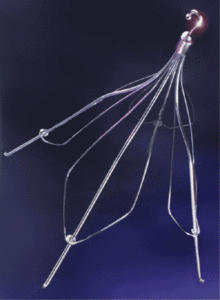
Cook Günther Tulip IVC Filter
The use of IVC filters has declined, but in 2007 — the year the Tulip was implanted in his body — they were more popular than ever.
Around 49,000 IVC filters were implanted in 1999. In 2003, Günther Tulip became one of the first FDA-approved retrievable IVC filters for short-term use. In 2007, around 167,000 IVC filters were implanted.
The popularity of IVC filters started declining in 2010, the same year the FDA issued a Safety Alert and several alarming safety studies were published.
The FDA received 921 injury reports from 2005 to 2010 and warned doctors against using retrievable filters as permanent implants.
One of the first alarming studies of IVC filters was published in 2010. Two of the most popular devices, the Bard Recovery and G2 IVC filters, were linked to high rates of fracture, embolization, and potentially life-threatening side effects. Bard stopped selling the G2 in 2010, after 160,000 were placed in patients.
A recent analysis of IVC filter placements from 1993 to 2010 shows a 9.36% year-over-year increase. Afterward, implants declined 7.36% per year.
The declining popularity of IVC filters had nothing to do with declining rates of blood clots or pulmonary embolisms. Those rates stayed constant, according to the author of the analysis, Dr. Ketan Petal.
The greatest decline was in the Northeast. The analysis also showed relatively conservative use of IVC filters in the West during the study period, contrasting with rampant use of IVC filters in Southern states.
Dr. Petal had no explanation for these geographical trends.
Another analysis by Dr. Vibhow Wadhwa at University of Arkansas showed that 2010 was the turning point in IVC filter use, as well as finding geographical discrepancies. Twice as many IVC filters were placed in the South compared to the West.
Many of those states now have a booming business in IVC filter removal, although the procedure is not without risks. A study by Dr. Jessica M. Andreoli found a 3.9% complication rate associated with the procedure. The longer a filter remains implanted, the higher the risk of complications.
Ironically, one of the most common long-term complications is blood clots (IVC thrombosis), the very same complication the filter was supposed to prevent. Experts now estimate that at least 2-4% of all cases of Deep Vein Thrombosis (DVT) are due to un-retrieved IVC filters.
Lawyers accuse Cook Medical of downplaying the risks associated with the Günther Tulip and selling a defective, dangerous device.
The lawsuit was filed on March 24, 2017 in the U.S. District Court for the Southern District of Indiana (Indianapolis Division) — Case No. 1:17-cv-00921.
It will be centralized with about 1,600 other IVC filter lawsuits now pending in Multi-District Litigation (MDL No. 2570) — In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation.
The plaintiff is represented by Ben C. Martin and Thomas Wm. Arbon of The Law Offices of Ben C. Martin.
Ben C. Martin is a trial attorney based in Dallas, Texas who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
