In a major reversal, the FDA has asked Allergan to recall BIOCELL® textured breast implants in the U.S. after finding 24 more deaths and a 6-fold increased risk of a rare but potentially deadly cancer.
Allergan had already recalled all of their textured breast implants in 38 countries several months ago, but they remained available in the U.S. while the FDA continued to investigate the safety risks.
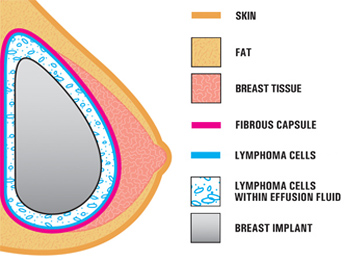
The FDA has spent the last 8 years studying the link between textured breast implants and Anaplastic Large Cell Lymphoma (ALCL), a rare type of blood cancer.
Now, the agency says it has found a “significant increase” in new cases of ALCL and deaths since 2018, when they refused to ban textured breast implants because they did not have enough data.
There are now a total of 573 known cases of breast implant-associated ALCL — including 481 attributed on Allergan’s breast implants — as well as 33 deaths worldwide.
Based on this data, the FDA said the risk of ALCL from Allergan’s BIOCELL® textured breast implants is approximately 6 times the risk of ALCL from textured breast implants sold by other manufacturers in the U.S.
Even so, the FDA does not recommend that patients undergo surgery to remove or replace the recalled breast implants, due to the risks of surgery.
Therefore, patients who have the implants should be vigilant for the symptoms of ALCL, such as persistent swelling or pain near the breast implant, and monitor their breasts for any unusual changes.
Allergan is now moving forward with a worldwide recall of their BIOCELL® textured breast implant products, including:
- Natrelle Saline-Filled breast implants
- Natrelle Silicone-Filled breast implants
- Natrelle Inspira Silicone-Filled breast implants
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants
U.S. healthcare providers with questions regarding this announcement can contact Allergan’s Medical Information at 1-800-678-1605 option #2 or IR-Medcom@allergan.com.