The lawsuit was filed by Maria A., a woman who was implanted with the C.R. Bard Meridian® Vena Cava Filter on November 8, 2012 at a hospital in California.
Meridian is a fifth-generation Bard IVC filter that was approved in 2011 and pulled off the market without a recall, like previous generations.
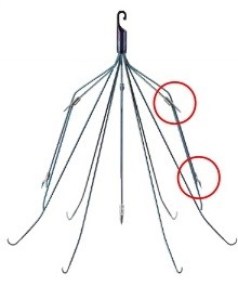
Bard Meridian® IVC Filter
Like these older filters, Meridian consists of 12 needle-like wires made of nitinol, a metal alloy made of nickel and titanium. The wires are arranged in an “umbrella” shape around a central hook.
The biggest difference between the Meridian and older Bard IVC filters is that some of the wires have sharp barbs near the hook that anchor it inside the inferior vena cava (IVC).
There were no changes in materials or manufacturing processes for Meridian filters compared to older products like the Recovery or G2.
While the barbs supposedly reduced the risk of Meridian filters tilting, the design changes did not address the most serious problems that plagued the Recovery or G2 — filter fracture and embolization.
Recent studies estimate that 40% of Recovery and G2 filters fracture in around 5 years. Fractures are blamed on metal fatigue due to nearly-constant flexing movements in the vein as it pulses with blood.
The most serious problems occur when the needle-like wires of an IVC filter break off and travel in the bloodstream until they hit the heart or lungs, resulting in life-threatening injuries or sudden death.
Fractures were frequently reported with the Recovery and G2, but less often with the Meridian. Even so, the FDA received 38 reports of spontaneous Meridian fractures in 2013 alone — including some patients who had broken pieces in their lungs, heart, or kidney vein.
In 2015, researchers published a case report of a 74 year-old woman who was severely injured when a Bard Meridian IVC filter fractured approximately 8-12 months after it was implanted in her body.
The researchers noted that Bard attempted to address these ongoing fracture concerns by replacing Meridian with the Denali IVC Filter, which has completely different materials and manufacturing process.
Lawyers accuse Bard of downplaying these risks, selling defective medical devices, failing to warn patients about side effects, and inadequately testing devices for safety before marketing them as safe.
The lawsuit was filed on January 20, 2017 in the U.S. District Court for the District of Arizona — Case No. 2:17-cv-00197.
It will be centralized with over 1,350 other IVC filter lawsuits now pending against C.R. Bard in Multi-District Litigation (MDL No. 2641) — In Re: Bard IVC Filters Products Liability Litigation.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas. He serves on the plaintiffs’ steering committee of the Bard IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

