The lawsuit was filed by Cheri Ann W., a woman from Kansas who was injured by the Meridian® Inferior Vena Cava Filter (“IVC Filter”) manufactured by C.R. Bard and Bard Peripheral Vascular Inc.
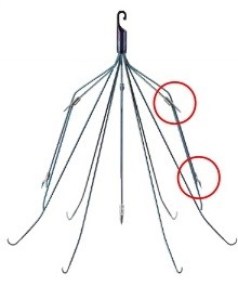
Bard Meridian® IVC Filter
The Meridian IVC filter was surgically implanted in her body at a hospital in Kansas on July 8, 2014 to prevent blood clot complications.
IVC filters are spider-like metal devices that catch blood clots floating through the largest vein in the body. They are supposed to reduce the patient’s risk of a deadly pulmonary embolism (blood clot in the lungs).
Unfortunately, thousands of people have suffered devastating IVC filter side effects when it broke apart or punctured through their vein.
The FDA issued a Safety Warning in August 2010 after receiving 921 injury reports from 2005 to 2010, of which 328 involved device migration, 146 involved embolizations (detachment of device components), 70 involved perforation of the vein, and 56 involved filter fracture. According to the agency:
These retrievable IVC filters, intended for short-term placement, are not always removed once a patient’s risk for PE subsides. Known long term risks associated with IVC filters include but are not limited to lower limb deep vein thrombosis (DVT), filter fracture, filter migration, filter embolization and IVC perforation.”
Lawsuits have been filed by over 3,000 people who were injured by many types of IVC filters. The manufacturers are all accused selling defective medical devices without providing adequate risk information.
The lawsuit was filed on April 18, 2017 in the U.S. District Court for the District of Arizona — Case No. 2:17-cv-01153.
C.R. Bard is currently facing around 1,700 other IVC filter lawsuits in a centralized federal Multi-District Litigation (MDL No. 2641) — In Re: Bard IVC Filters Products Liability Litigation.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas. He serves on the plaintiffs’ steering committee of the Bard IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
