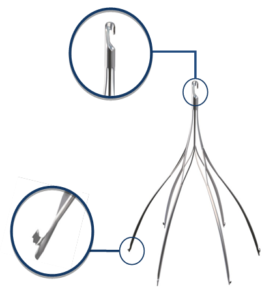
Argon Option ELITE Inferior Vena Cava (IVC) Filter
The lawsuit was filed by Ronald D., a man from South Carolina who was implanted with the Option™ ELITE Retrievable Inferior Vena Cava on April 19, 2015 at University Hospital in Augusta, Georgia.
The same doctor tried to remove the filter on April 12, 2016. At that time, he discovered the filter was tilted 45º and the retrieval hook was embedded in the wall of the vein. Despite a complex procedure, the filter could not be removed.
On June 29, 2016, Ronald went to another doctor to remove the filter. The procedure was successful, but there were serious consequences. According to the complaint:
The doctor was eventually able to successfully remove the filter. After removal of the IVC filter, it was discovered that the IVC wall was damaged where the filter hook had been embedded.”
The inferior vena cava (IVC) is the largest vein in the body. It carries “used” blood from the lower half of the body to the lungs and heart to be re-oxygenated and pumped back to the body.
Damage to the wall of the IVC is a serious complication of removing embedded filters because it can potentially cause major internal bleeding or death. Many doctors will not take the risk of removing an embedded filters.
The problem is that the risk of other serious complications increases the longer the filter remains implanted. It is why the FDA recommends removing temporary filters like the Option ELITE within 29-54 days, ideally.
The FDA approved the Option ELITE filter in December 2013 through the 510(k) process, which allows new devices on the market without clinical trials so long as the device is “equivalent” to another device.
Option ELITE was “equivalent” to the original Option, which was itself “equivalent” to the Bard Recovery, G2, Cook Gunther Tulip, and more. All of these other filters were linked to serious safety hazards long before the Option ELITE filter was approved. The FDA issued a Safety Warning in 2010.
Lawyers say Argon Medical knew or should have anticipated similar problems with the Option ELITE, but continued manufacturing and selling a defective medical device with unreasonably dangerous risks.
Argon Medical is accused of failing to warn patients and doctors about dangerous side effects, such as hemorrhage, thrombosis, cardiac/pericardial tamponade, cardiac arrhythmia and other symptoms similar to a heart attack, perforations of the vein and organs, and death.
The lawsuit was filed on March 14, 2017 in the Philadelphia Court of Common Pleas — Case ID: 170301260.
There are currently over 3,000 other IVC filter lawsuits pending against Argon Medical Devices, C.R. Bard, and Cook Medical. Most lawsuits have been centralized in federal court in Arizona and Illinois.
The plaintiff is represented by attorney Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas; and attorney Stephen A. Sheller of Sheller, P.C., in Philadelphia, Pennsylvania.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

My brother has had two surgery’s and they have not been able to remove it. It was installed in 2016 by San Diego Hospital and it’s still in his body. My brother leg is swollen and he also have issues breathing, please call him. Thank you so much