The lawsuit was filed by Jeffrey Z., a man who was implanted with the Cook Günther Tulip® IVC Filter on November 11, 2009 at St. Joseph Medical Center in Brainerd, Minnesota.
Cook Medical developed the Tulip as a permanent filter the 1980s, but it was not widely-used until the FDA approved it for retrieval in 2003.
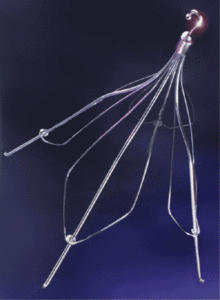
Cook Günther Tulip IVC Filter
Before 2003, the Tulip and other permanent IVC filters were primarily used as a “last resort” for patients who were diagnosed with blood clots, and only if they could not take a blood-thinning drug instead.
That all changed in the mid-2000s, when the FDA approved a variety of temporary IVC filters. They were routinely implanted in patients without any blood clots, even patients who could take blood-thinners.
Manufacturers claimed the temporary filters provided all the benefit of blood clot protection without the commitment of a notoriously risky permanent IVC filter — making them ideal for a wide range of patients.
The result can be seen in statistics. Approximately 49,000 permanent IVC filters were implanted in 1999. That number jumped 5-fold to around 250,000 by 2012, mostly due to the use of temporary filters.
There was little evidence they worked and no long-term safety studies, but only 30% were ever removed. Complication rates also increase over time, which is why most should be taken out within 29-54 days.
When the FDA became concerned that doctors were using temporary filters like permanent implants in 2010, the agency issued a Safety Alert about long-term complications of temporary IVC filters.
The most common complication reported to the FDA was migration (tilting or moving out of position), followed by embolizations (detachment of filter components) and perforations of the vein.
Tilting and vein perforations are commonly associated with the Tulip. In a study from 2007, tilting was why 24% of Tulip filters could not be removed after being implanted for at least 180 days.
In a study from 2013, Tulips caused perforations in 43% of patients within 14 months, on average. In 2012, a study showed that all Tulip filters had “some degree” of perforation within 71 days, often as a progressive process.
Lawyers say Cook Medical did not warn patients about the risk of severe side effects. The company is also accused of aggressively marketing defective medical devices without adequate safety tests.
The lawsuit was filed on January 12, 2017 in the U.S. District Court for the Southern District of Indiana (Indianapolis Division) — Case No. 1:17-cv-00130.
It will be centralized with over 1,370 other IVC filter lawsuits now pending in Multi-District Litigation (MDL No. 2570) — In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation.
The plaintiff is represented by Ben C. Martin and Thomas Wm. Arbon of The Law Offices of Ben C. Martin.
Ben C. Martin is a trial attorney based in Dallas, Texas who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

