The lawsuit was filed by Judith S., a woman who was implanted with the Cook Günther Tulip® Vena Cava Filter on June 6, 2016 at Rhode Island Hospital in Providence.
Cook Medical has sold the Tulip as a permanent implant since the 1990s. It did not become widely-used until after 2003, when it was approved for retrieval and short-term protection against blood clots.
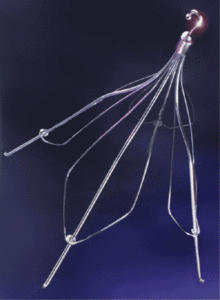
Cook Günther Tulip IVC Filter
Blood clot filters were never popular as permanent implants due to the risk of major side effects. Retrievable filters seemed to be the answer — but the problem is that only about 30% were ever actually retrieved.
The FDA received 921 injury reports between 2005 and 2010 and warned that retrievable filters should not be used as permanent implants.
The top complaints to the FDA were migration (tilting or movement out of position), embolization of broken components, and vein perforation.
Tilting is a common problem with the Günther Tulip. In one study of 41 patients who were implanted with a Tulip filter for at least 180 days, 24% could not be removed because they were tilted.
Another common problem is vein perforations — 43% of Tulip filters caused a perforation within 14 months, according to a study in 2013. In another study, all 23 patients with the Tulip developed “some degree” of vein perforation within 71 days.
The inferior vena cava (IVC) is the largest vein in the human body, but its walls are incredibly thin due to the low pressure exerted by venous blood.
The needle-like legs of the Tulip dig into the walls of this vein to anchor the filter in place. Over time, the legs can dig too deep and puncture through the vein, causing the whole filter to tilt.
Perforations and tilting do not always cause symptoms, but they usually make the filter harder to remove — or impossible, if the hook on the tip of the filter becomes embedded in the vein.
Blood clots are another risk. Tilted filters may not effectively trap blood clots. Instead, they may actually lead to the development of new blood clots — a complication called IVC thrombosis.
Many lawsuits have been filed by people who will have a “temporary” IVC filter in their body for the rest of their life. Cook Medical is accused of failing to warn about the risk of side effects.
The lawsuit was filed on January 12, 2017 in the U.S. District Court for the Southern District of Indiana (Indianapolis Division) — Case No. 1:17-cv-00115.
It will be centralized with over 1,370 other IVC filter lawsuits now pending in Multi-District Litigation (MDL No. 2570) — In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation.
The plaintiff is represented by Ben C. Martin and Thomas Wm. Arbon of The Law Offices of Ben C. Martin.
Ben C. Martin is a trial attorney based in Dallas, Texas who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

