The FDA issued a Class 1 recall for the Endologix Ovation iX Abdominal Stent Graft System due to a risk of severe injury or death.
The recall involves about 5,403 devices that were distributed in the U.S. since August 2015.
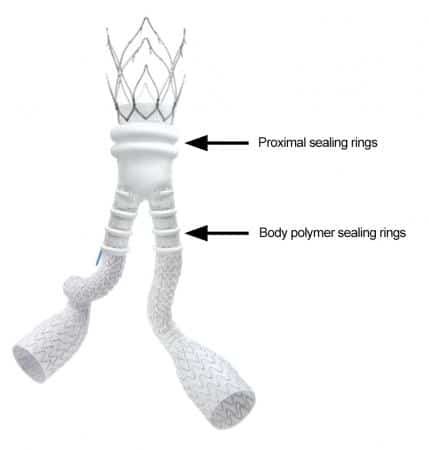
The stent is used to repair abdominal aortic aneurysms, a condition that occurs when the body’s largest blood vessel (the aorta) develops a weak spot that bulges outward until it suddenly bursts open.
Unlike traditional stent grafts, Ovation iX contains a liquid polymer. Unfortunately, the liquid polymer can potentially leak out of the graft and cause severe, life-threatening complications.
In an update on June 15, Endologix said 112 patients had suffered adverse events due to the polymer leakage problem, including 5 patients who died.
Some of the most serious adverse events include sudden low blood pressure, severe hypersensitivity reactions, multi-organ failure, cardiac arrest, and tissue necrosis.
Endologix first reported the polymer leaking problem in 2018. At the time, the company blamed the problem on user error.
Now, Endologix believes the leaks are due to a “material weakness” next to the polymer channel. Unfortunately, another 65 patients were injured due to the polymer leak problem during that time.
Endologix is asking doctors to transition to another stent graft called Alto, which was redesigned to fix problems with material weaknesses. CEO John Onopchenko said this should “meaningfully decrease polymer leak rates compared to Ovation iX.”