Brassica Pharma Pvt. Ltd. has recalled four types of eye ointments after FDA inspections found evidence of insanitary conditions.
The recall involves the following brands of eye ointments:
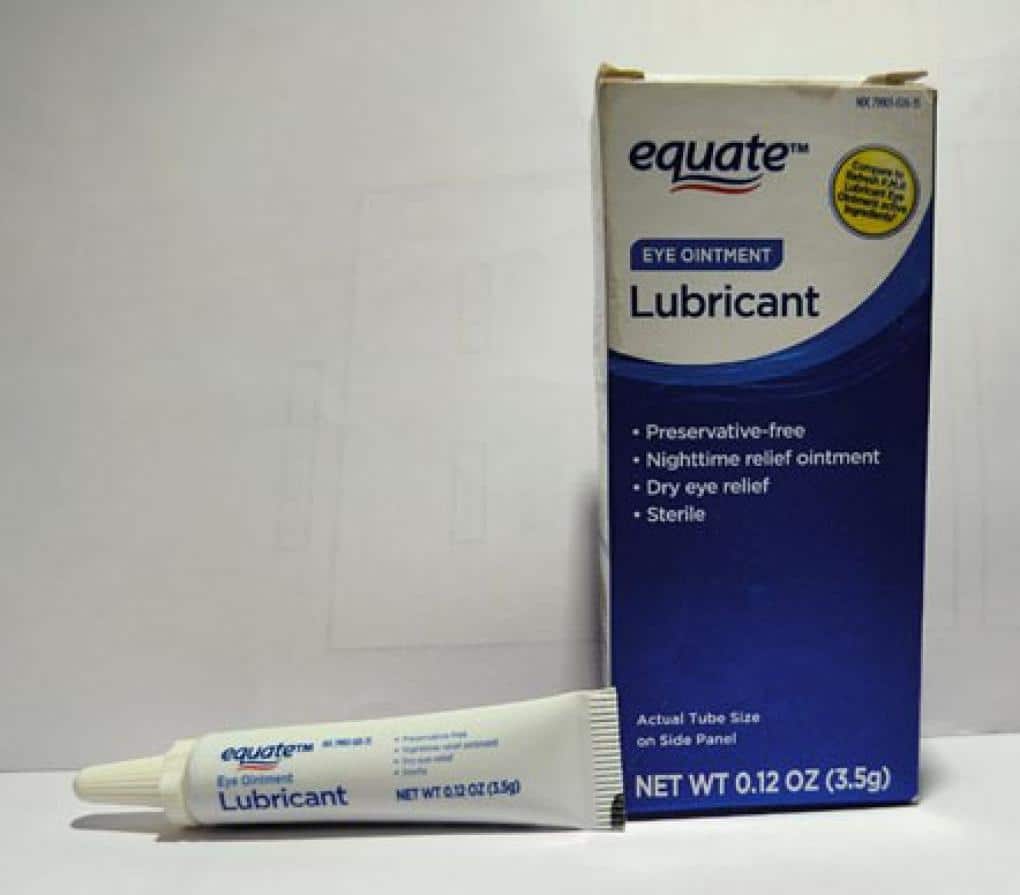
- Equate Lubricant Eye Ointment (Mineral Oil 42.5%, White Petrolatum 57.3%, Lanolin Alcohols) — 3.5 gram tube, packaged in cardboard box with UPC Code 681131395298
- Equate Stye Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid, Wheat Germ Oil) — 3.5 gram tube, packaged in cardboard box with UPC Code 681131395304
- CVS Health Lubricant Eye Ointment (Mineral Oil 31.9%, White Petrolatum 57.7%, Microcrystalline Wax, Stearic Acid Wheat Germ Oil — 3.5 gram tube, packaged in cardboard box with UPC Code 050428634141
- AACE Pharmaceuticals Lubricant PM Ointment — 3.5 gram tube, packaged in cardboard box with UPC Code 371406124356
These recalled eye ointments were sold nationwide at Walmart stores, CVS, AACE Pharmaceuticals Inc., and distributors.
No injuries were reported, but an FDA inspection noted a “lack of sterility assurance at the facility” in India. The FDA has stepped up enforcement of Indian drug-makers after a deadly outbreak of infections in 2023 was linked to eye drops manufactured in India.
Brassica Pharma Pvt. Ltd. is asking customers to stop using the recalled eye ointments and return the products to the place of purchase. “Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product.”