The lawsuit was filed by Gail P., a woman from California who was implanted with the Cook Günther Tulip® Vena Cava Filter on October 2, 2007 at Stanford Hospital.
Günther Tulip has been on the market for decades — since 1992 in Europe and 2000 in the U.S. — and it became one of the first IVC filters to be approved for short-term implantation by the FDA in 2003.
The FDA was quick to approve the Tulip for retrieval because it was already being used for that purpose. Cook Medical designed it with a hook on one end, allowing doctors to lasso it and pull it out of the vein.
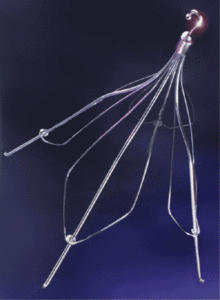
Cook Günther Tulip IVC Filter
It was also designed with needle-like wires arranged into four “petals,” like a Tulip, that dig into the vein and anchor the filter in place. Over time, it can dig deeper or puncture the vein and into nearby organs.
In one recent study, all patients with the Tulip had “some degree” of perforation within 71 days, often as a progressive process. In another study, 43% of Tulip IVC filters caused a perforation within 14 months.
Tilting is another common problem. In a study of 41 patients, 24% of Tulip filters were impossible to remove because they developed mild-to-severe tilting within 180 days (6 months).
These risks were a big reason why permanent filters were only used in specific circumstances — mostly hospitalized patients who were diagnosed with blood clots, but could not take blood-thinning drugs.
When temporary IVC filters were approved in the mid-2000s, hospitals started routinely implanting them in patients at risk of blood clots — including patients without clots who could take blood-thinners instead.
Less than 50% of these filters were ever removed, which means tens of thousands of Americans have a “ticking time bomb” in their body. Ironically, one of the most common long-term side effects is developing blood clots due to the filter causing circulation problems.
Cook Medical is accused of aggressively selling a dangerous medical device, downplaying side effects, and inadequately testing it for safety.
The lawsuit was filed on January 12, 2017 in the U.S. District Court for the Southern District of Indiana (Indianapolis Division) — Case No. 1:17-cv-00112.
It will be centralized with over, 1,370 other IVC filter lawsuits now pending in Multi-District Litigation (MDL No. 2570) — In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation.
The plaintiff is represented by Ben C. Martin and Thomas Wm. Arbon of The Law Offices of Ben C. Martin.
Ben C. Martin is a trial attorney based in Dallas, Texas who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.

