One lawsuit was filed by Susan M., a woman who was implanted with the Cook Günther Tulip® Vena Cava Filter on February 28, 2006 at Evergreen Hospital Medical Center in Kirkland, Washington.
The Günther Tulip is a permanent filter that is implanted in a vein called the inferior vena cava (IVC) to catch blood clots. It has a hook to permit retrieval, allowing doctors to lasso the filter and remove it from the vein when the patient no longer needs it.
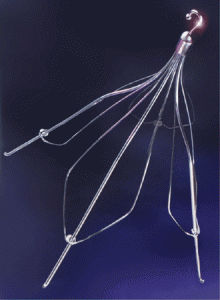
Günther Tulip Vena Cava Filter
In October 2003, the Tulip became one of the first IVC filters approved by the FDA for retrievable use. This vastly expanded the use of the Tulip filter in hospitals, especially in trauma patients without blood clots. It also created problems because less than 50% were actually retrieved.
The problems include filter legs puncturing through the vein and into nearby organs, severe tilting causing filter retrieval failure, and filters fracturing and traveling in the bloodstream until they damage the heart or lungs.
In recent years, several studies have linked the Günther Tulip with high rates of vein penetration and other side effects, with the risk increasing the longer the Tulip remains implanted in a patient.
For example, a study titled “Perforation of the IVC: Rule rather than exception after longer indwelling times for the Günther Tulip” found that all patients had “some degree” of perforation within 71 days, often as a progressive process. Many filters were also tilted.
In 2013, another study found that 69 of 160 Tulip filters (43%) punctured the vein within 14 months, on average. In comparison, only 1 of 50 Greenfield filters (2%) punctured the vein within 9 months.
Vein perforations do not usually cause symptoms, so patients may not know they have a problem until it causes catastrophic complications. There are case reports of people who needed emergency open-heart surgery when the metal legs of a Günther Tulip IVC Filter broke off and punctured their heart.
Cook Medical is facing a growing number of lawsuits from people who say the company sold a dangerous device, downplayed risks, failed to warn about side effects, and never adequately tested it for safety.
The lawsuit was filed on January 26, 2017 in the U.S. District Court for the Southern District of Indiana (Indianapolis Division) — Case No. 1:17-cv-00256.
It will be centralized with over 1,370 other IVC filter lawsuits now pending in Multi-District Litigation (MDL No. 2570) — In Re: Cook Medical, Inc., IVC Filters Marketing, Sales Practices, and Products Liability Litigation.
The plaintiff is represented by Ben C. Martin and Thomas Wm. Arbon of The Law Offices of Ben C. Martin.
Ben C. Martin is a trial attorney based in Dallas, Texas who serves as the plaintiffs’ co-lead counsel in the Cook IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.